Background:The CAR structure adopted by CART-BCMA is composed of a single-domain antibody targeting BCMA, CD8 hinge region, transmembrane region, 4-1BB co-stimulatory domain, and CD3-ζ T cell activation domain. In the phase I clinical study (NCT05346198) carried out in China, the safety and efficacy of CART-BCMA were initially evaluated through the dose escalation + dose expansion design, and the recommended dose (RD dose) for follow-up studies was determined as well.
Method:Enrolled patients with relapsed and/or refractory multiple myeloma (RRMM) had previously received at least 3 lines of therapy including at least one proteasome inhibitor and one immune modulator. After lymphatic preconditioning chemotherapy (program: fludarabine 30mg/m 2/d and cyclophosphamide 300mg/m 2/d, -5, -4, -3, for 3 consecutive days), all patients received a single dose of CART-BCMA at 2.5×10 6, 5×10 6 and 7.5×10 6 CAR-positive T cells/kg (subject body weight), according to the dose level in which they were enrolled.
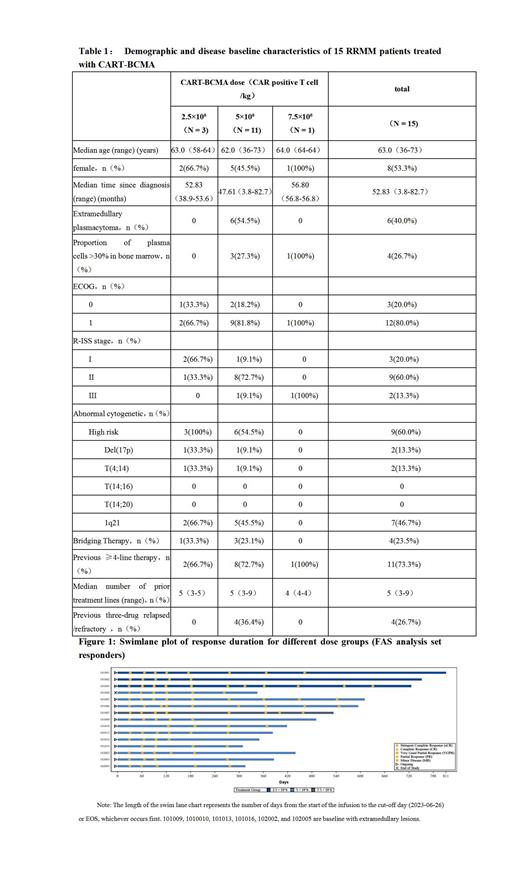
R esults :Up to June 26, 2023, a total of 15 cases of RRMM received 2.5×10 6 CAR-positive T cells/kg (n=3), 5×10 6 CAR-positive T cells/kg (n=11) or 7.5×10 6 CAR-positive T cells/kg (n=1) dose level of CART-BCMA cell infusion therapy on the basis of the dose level in which they were enrolled. The median follow-up time was 14.42 months (range: 10.2-26.6 months). The median age of the subjects was 63.0 years old (range: 36-73 years old) (Table 1), 73.3% of the subjects (n=11) had previously received ≥4 lines of multiple myeloma therapy, and the median line of treatment was 5 lines (range: 3-9 lines), among which, 2 subjects (13.3%) had previously received autologous hematopoietic stem cell transplantation, and 4 subjects (26.7%) had relapsed/refractory diseases after previous treatment with proteasome inhibitors, immune modulators and CD38 monoclonal antibodies (three-drug relapsed/refractory). Nine subjects (60.0%) had high-risk cytogenetic abnormalities according to sMART 3.0 criteria, and 6 subjects (40.0%) had extramedullary plasmacytoma.
The most common grade ≥3 adverse events were hematological toxicity, including decreased neutrophil count (n=13, 86.7%), decreased white blood cell count (n=12, 80.0%), decreased lymphocyte count (n=10, 66.7%), anemia (n=8, 53.3%), decreased platelet count (n=4, 26.7%). The first patient in the 7.5×10 6 CAR-positive T cells/kg dose group had a dose-limiting toxicity (DLT) event of grade 4 platelet count decrease, and no DLT event occurred in the other low-dose groups (2.5 and 5×10 6 CAR-positive T cells/kg). All 15 patients (100.0%) developed grade 1-2 cytokine release syndrome (CRS), no grade ≥3 CRS occurred, and the median time to CRS was 2.0 days (range: 1-13 days) , with a median duration of 7.0 days (range: 2 to 17 days). After the occurrence of CRS, all patients recovered without sequelae after supportive treatment, and 6 patients (40%) received tocilizumab (3 subjects received 2 doses, 3 subjects received 1 dose), 4 patients (26.7%) used glucocorticoids. There was no event of immune effector cell-associated neurotoxicity syndrome (ICANS), and no death event occurred.
The median time to the first response (TTR) of the patients was 0.953 months (range: 0.92 to 2.23 months). All 15 patients who received CART-BCMA infusion achieved remission, and the overall remission rate (ORR) ( At least achieve PR or better efficacy) was 100% (95%CI, 78.20%-100.00%), as shown in Figure 1, among which, the best response of 9 patients (60%) was strict complete remission ( sCR), 5 patients (33.3%) achieved very good partial response (VGPR), and 1 patient (6.7%) achieved PR. All sCR patients achieved MRD negativity. The ORR of 6 patients with extramedullary plasmacytoma was 100%, of which 2 achieved sCR and 4 achieved VGPR. Notably, gradual deepening of remission was observed in 12 patients (80%), of which 10 patients (66.7%) further deepened the depth of remission on days 60-90 after infusion, and 2 patients (13.3%) were assessed sCR at day 360.
Conclusion:Data from this phase 1 clinical study showed that CART-BCMA is well tolerated and highly efficacious in patients with RRMM. Patients with extramedullary plasmacytoma had the comparable response to those without extramedullary diseases. Notably, 12 patients (80%) patients elicted deeper response after 3 months of CART-BCMA infusion
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal